Sex Hormones – Testosterone, Progesterone, and Oestrogens
Sex hormones such as testosterone, progesterone, and oestrogens play a central role in regulating numerous bodily functions. Testosterone, primarily produced in the Leydig cells of the testes, is responsible for the development of male sex organs and anabolic cell growth. Smaller amounts are also formed in the adrenal glands and female ovaries. An imbalance in testosterone production can lead to various health issues.
Oestrogens, mainly produced in the ovaries, are essential for the regulation of the female reproductive system and the development of secondary sexual characteristics. They also influence bone metabolism and the cardiovascular system. An oestrogen imbalance can similarly impact health.
Progesterone, a representative of the gestagens, is mainly produced in the corpus luteum of the ovaries and plays a key role in preparing and maintaining pregnancy. It also influences thyroid function and has extra-genital effects such as stabilising the central nervous system. A deficiency in progesterone can lead to symptoms such as premenstrual syndrome, menstrual disorders, and sleep disturbances.
A delicate hormonal balance is essential for the health of both sexes. Disruptions in hormone production can result in androgen- or oestrogen-dependent diseases. Therefore, precise laboratory diagnostics are important to assess hormonal status.
Testosterone – The “Male Hormone”
The term androgens refers to the group of male sex hormones. The best-known of these is testosterone, which acts as an endocrine messenger regulating various bodily functions. This hormone ensures the functionality and development of male reproductive organs and is responsible for anabolic cell growth. Production primarily occurs in the Leydig cells of the testes under the influence of LH (luteinising hormone) and is transported via the bloodstream to target organs such as the reproductive organs, skin, muscle and fat tissue, bones, liver, and prostate. However, testosterone production is not confined to the male gonads: small quantities are also formed in the adrenal glands—both in men and women—and in the female ovaries. Detectable serum concentrations in women are only a fraction of those in men. After reaching the target organs, testosterone acts either as an independent hormone or as a precursor to further bioactive metabolites, each with specific physiological functions in a finely tuned balance (see Figures 1 and 2). This balance is determined by the activity and quantity of specific enzymes. If their production is disrupted, this can lead to hormonal dysregulation, potentially causing androgen- or oestrogen-related diseases in both sexes.
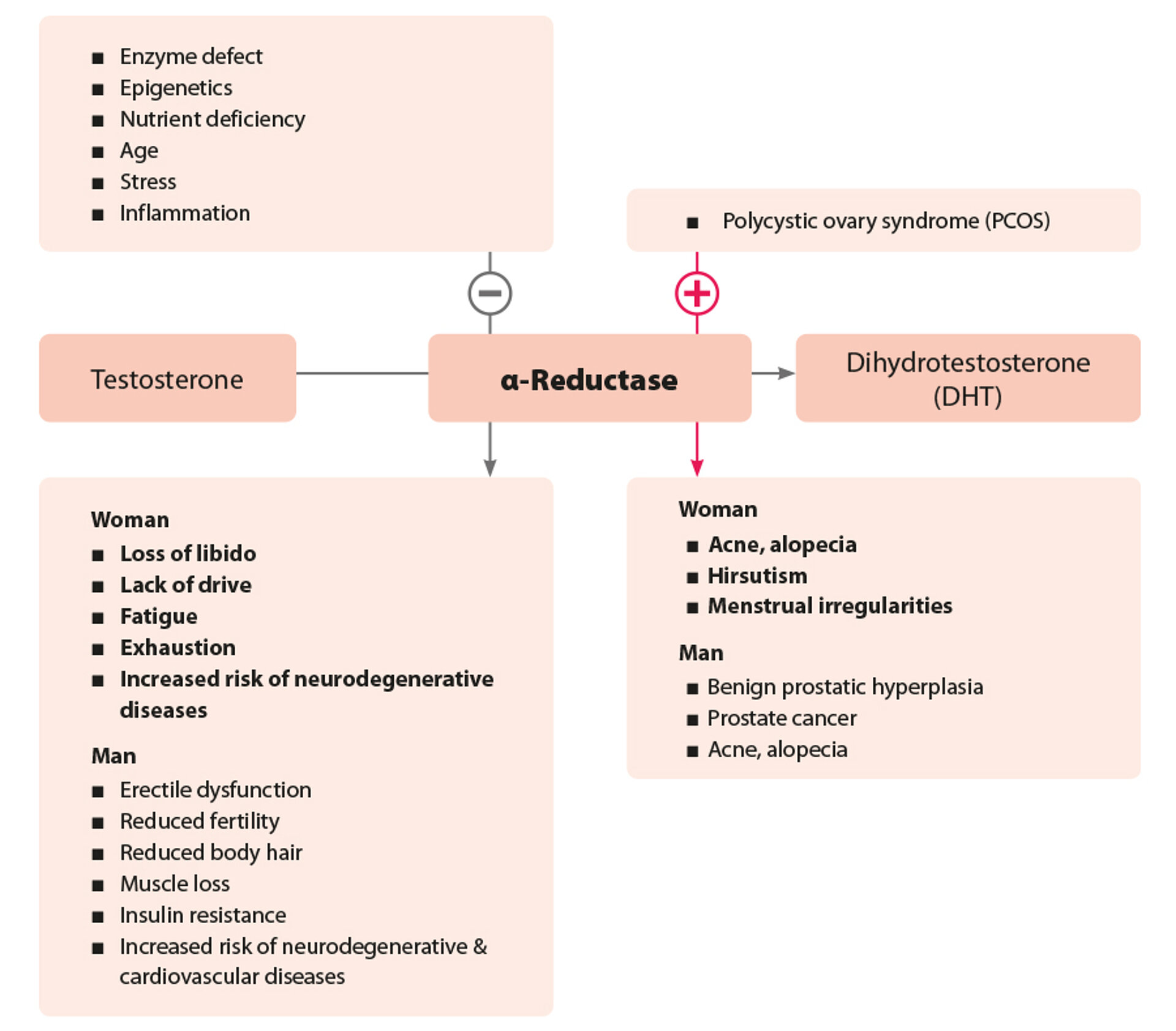
The Enzyme 5α-Reductase Potentiates Testosterone Activity
In the male body, the enzyme 5α-reductase typically dominates. It can amplify the androgenic effect of testosterone tenfold. Therefore, 5α-reductase activity essentially determines the function of male reproductive organs, male body morphology, and various aspects of metabolism such as fat and glucose metabolism. The activity of 5α-reductase can be determined in the laboratory by measuring the testosterone to dihydrotestosterone ratio in the blood.
Consequences of 5α-Reductase Hypoactivity
Reduced 5α-reductase activity in men leads to androgen deficiency, which may manifest as erectile dysfunction, decreased libido, fertility issues, and reduced body hair. Additionally, androgen deficiency is associated with muscle loss and an increased risk of insulin resistance and cardiovascular disease. In women, androgen deficiency typically presents as loss of libido, fatigue, lack of drive, and exhaustion. Both sexes are also at a higher risk for neurodegenerative diseases.
Consequences of 5α-Reductase Hyperactivity
Hyperactivity of 5α-reductase in men promotes the development of benign prostatic hyperplasia, potentially leading to dysfunctions in the lower urogenital tract. It is also associated with acne and androgenic alopecia. Women can also be affected, with symptoms including acne and progressive scalp hair loss. In addition, hyperactivity can lead to hirsutism (excessive facial hair) and menstrual disorders such as polycystic ovary syndrome (PCOS).
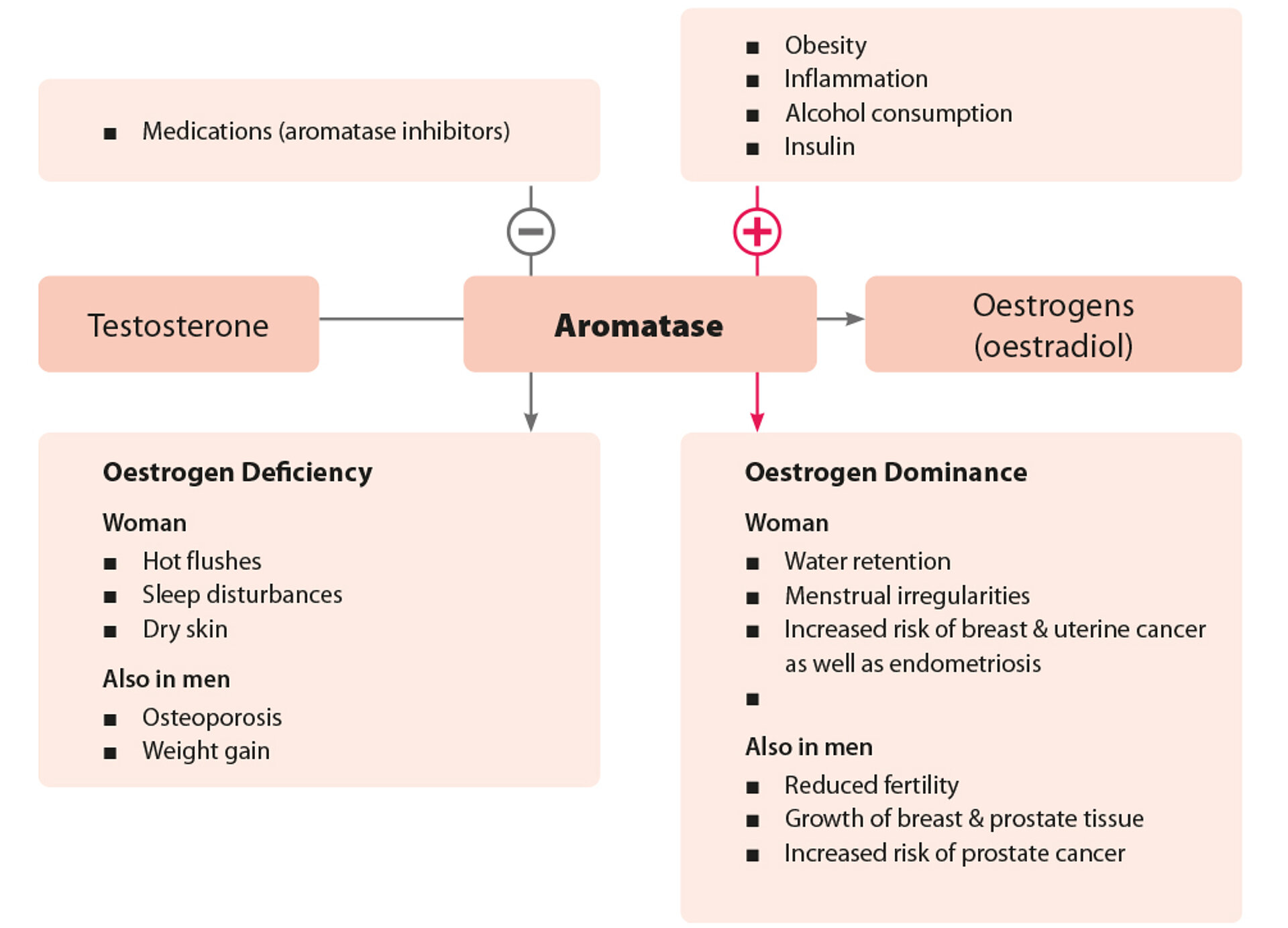
The Enzyme Aromatase Reduces Testosterone Activity
The physiologically low testosterone serum level in women is due to the dominant aromatase-mediated breakdown of testosterone into oestradiol. In addition to the already low testosterone production in women, its androgenic effect is further diminished by this conversion. Aromatase activity can be calculated by determining the testosterone/oestradiol ratio in urine.
Consequences of Aromatase Hyperactivity
In men, increased oestrogen production can impair fertility. It may also lead to gynaecomastia (breast tissue growth) or prostate enlargement. Women may experience symptoms such as water retention and menstrual irregularities. Excess oestrogen also increases the risk of uterine cancer and endometriosis.
Consequences of Aromatase Hypoactivity
Reduced aromatase activity is rare. In women, resulting oestrogen deficiency tends to be more pronounced than in men and resembles menopausal symptoms, including temperature regulation disorders and sleep disturbances. In addition, sebum and sweat gland activity are reduced, leading to dry skin and mucous membranes.
Oestrogens – Female Sex Hormones
Oestrogens are steroid hormones with 18 carbon atoms, synthesised from cholesterol via androgen precursors primarily in the ovaries. In postmenopausal and obese women, oestrogens are increasingly produced in fat and muscle cells through elevated aromatase (CYP19) activity. In the blood, they are largely bound to globulins (sex hormone-binding globulin = SHBG) and albumin, with only 1–2% circulating in free form. A premenopausal woman produces between 30 and 300 µg of oestradiol (E2) daily, depending on the menstrual cycle. The majority is converted to the metabolite oestrone (E1) or, during pregnancy, to oestriol (E3). Oestrogen metabolites can be biochemically classified as either protective or potentially mitogenic/mutagenic.
Carcinogens are substances capable of altering cellular DNA (tumour initiation) and promoting cell proliferation (tumour promotion). Some oestrogen metabolites possess these properties.
Men produce around 20–50 µg of oestradiol daily, with levels remaining relatively constant into old age. In comparison to fertile men, subfertile men often exhibit reduced oestradiol levels. Assessing the full oestrogen metabolism allows for the evaluation of potentially carcinogenic vs. protective oestrogens, offering insight into an individual's potential risk. This assessment is not intended to provide a definitive cancer prognosis but to help healthcare providers identify risk factors early and develop preventative strategies with patients.
Progesterone – A Representative of the Gestagens
Overview of Progesterone
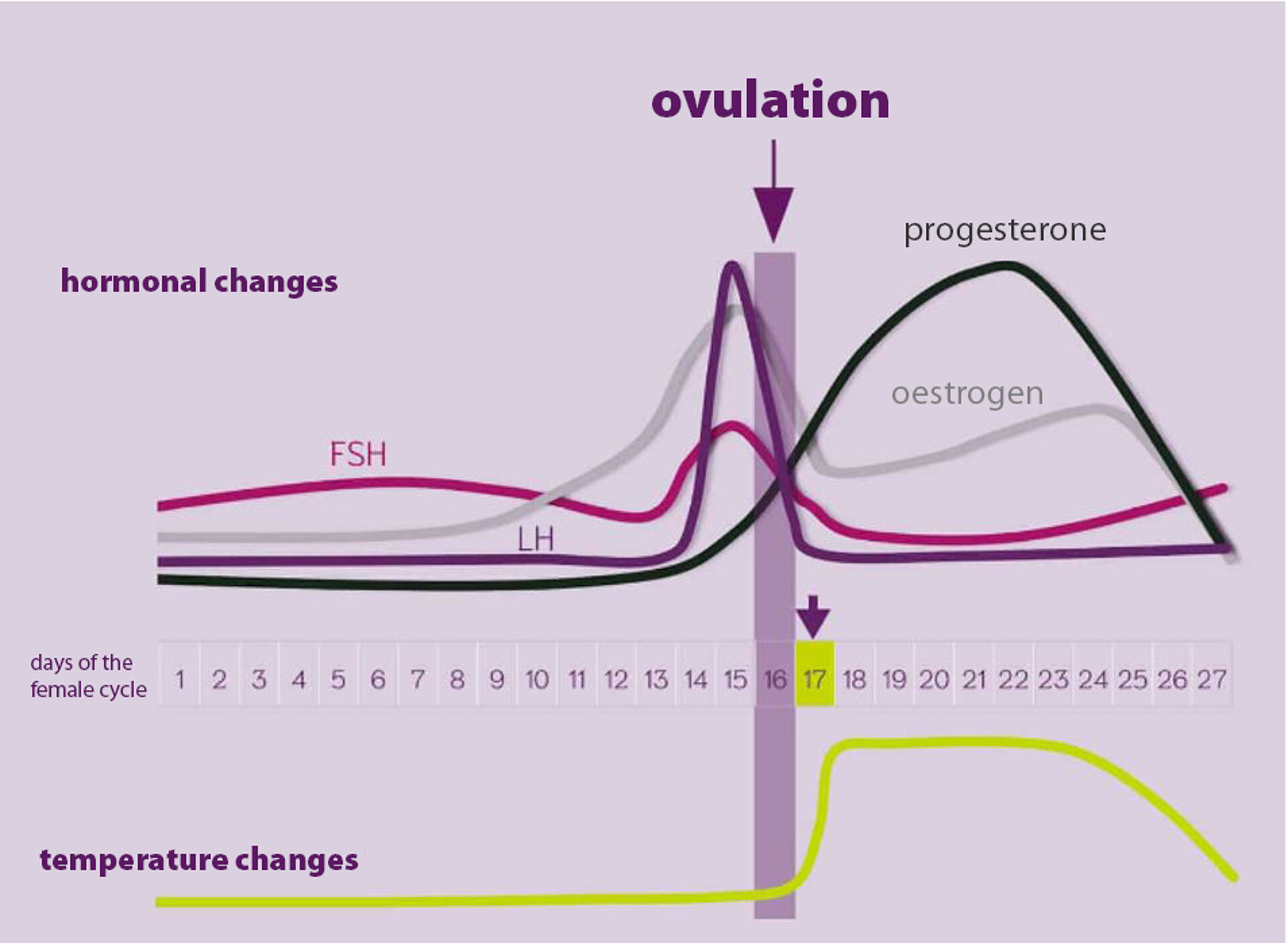
Progesterone interacts with oestrogens in regulating nearly all female reproductive functions. Its primary role is to prepare the uterus for implantation and support the pregnancy by suppressing uterine contractions and modulating immune response. If fertilisation does not occur, the corpus luteum (main site of progesterone production) regresses, and progesterone levels sharply decline approximately three days before menstruation. This physiological drop is often linked to depressive symptoms of premenstrual syndrome.
Beyond reproductive organs, progesterone also affects thyroid function by stimulating thyroperoxidase activity, which leads to increased thyroid hormone production. This can be observed as a post-ovulatory rise in basal body temperature (thermogenic effect). Tracking the basal temperature curve can help assess progesterone levels and confirm ovulation. Conversely, low progesterone levels may reduce thyroid hormone levels.
Extragenital Effects of Progesterone
The effects of progesterone extend well beyond pregnancy maintenance. It also has diuretic, blood pressure-lowering, and antithrombotic effects. Notably, it stabilises the central nervous system, with the metabolite allopregnanolone exhibiting antidepressant and neuropsychiatric stabilising properties.
Progesterone Deficiency
Symptoms of low progesterone levels do not necessarily indicate an absolute deficiency. Many women experience an imbalance between oestrogen and progesterone, known as oestrogen dominance, where excessive oestrogen causes the symptoms. Common symptoms associated with progesterone deficiency (or oestrogen dominance) include:
- Premenstrual syndrome (PMS)
- Menstrual and cycle irregularities (spotting, polymenorrhoea, short cycles)
- Tendency towards oedema (especially in the face and hands) / weight gain
- Sweating (night and day)
- Sleep disturbances
- Depressive moods
- Nervousness, restlessness
- Reduced libido
- Thyroid dysfunction
- Anxiety
- Venous weakness
Tumour Protection vs. Tumour Initiation by Progesterone
Many refer to older studies (e.g., early 1990s) when assessing progesterone’s pharmacological profile, which rarely mention adverse effects or tumour risks. However, more recent data indicate that even natural progesterone must be evaluated critically. As with all potent biological agents, significant effects can entail side effects.
In gynaecology, natural progesterone is used to protect the endometrium during oestrogen therapy, particularly in postmenopausal women. Oestrogen-induced cell proliferation (as part of hormone replacement therapy) increases the risk of oestrogen-associated tumours, which progesterone helps to counteract. Hence, combining oestrogen and progesterone has become standard in HRT, reducing proliferative pressure on hormone-sensitive tissues like the endometrium. However, the tumour-protective effect depends on the tissue’s specific enzyme profile and does not apply to breast tissue. Recent studies show that progesterone may increase the risk of breast cancer, accelerate tumour growth, and promote metastasis.
After age 52, corpus luteum formation ceases, and progesterone levels remain low. The adrenal glands continue to produce some progesterone, but oestrogen production persists despite ovarian failure. As a result, the loss of progesterone’s protective effects increases the risk of hormone-dependent tumours.